
The Foundation of Quality Starting Materials Begins with Standardized Apheresis
The promise of cell therapies to transform patient outcomes is matched only by the complexity of their supply chains, which begin with a single, critical step.
The collection of starting materials.
Yet in our conversations with clients and partners across the cell therapy industry, there's a troubling disconnect between this promise and current reality. Organizations consistently share the same fundamental apheresis challenges: center qualification and auditing, protocol standardization across sites, collection quality and consistency, managing regulatory compliance across regions, and patient scheduling and availability.
These aren't separate problems but symptoms of the same underlying issue. While the cell therapy industry has made remarkable advances in manufacturing science and clinical applications, the foundation of it all, standardized, high-quality starting material collection, remains fragmented across hundreds of individual centers, each operating with different protocols, equipment, and training standards.
We all know how high the stakes are. Every collection represents a patient waiting for treatment, a clinical trial timeline, and millions of dollars in development investment. It's clear that the industry's next evolutionary step won't come from the lab but will come from standardizing apheresis.
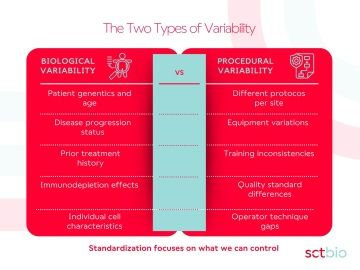
The Two Types of Variability
Not all variability is created equal. In apheresis collections, we face two distinct types of variation, and understanding the difference is crucial for developing effective solutions.
Biological variability is inherent to the biology we're working with. Patient-to-patient differences in cell quality and quantity are inevitable consequences of individual genetics, age, and health status. Patients who have undergone prior treatment regimens present unique challenges that no amount of protocol standardization can fully eliminate.Procedural variability, however, is entirely preventable. Across the industry, organizations struggle with protocol standardization across sites, center qualification and auditing processes, and managing regulatory compliance across regions. Different protocols across collection sites, equipment differences, operator training gaps, and inconsistent quality standards all represent unnecessary sources of variation.
Consider this: if you're working with 50 different apheresis centers across multiple countries, each with their own protocols and equipment preferences, you're not managing a supply chain but managing 50 different experiments.
The critical distinction: While biological variability will always exist, procedural standardization represents our greatest opportunity for improvement.
The Real Cost of Inconsistency
When procedural variability compounds biological challenges, the impact extends far beyond collection statistics. Our clients frequently report manufacturing delays directly attributable to starting material inconsistencies, with batch complications that could have been prevented through standardized protocols.
Perhaps most concerning: starting material inconsistencies directly impact patient treatment timelines. In our experience working with autologous therapy developers, where patients are often critically ill, these delays aren't just operational inconveniences but can affect clinical outcomes and quality of life.
The Standardization Solution
Addressing procedural variability requires systematic approaches that acknowledge biological realities while eliminating unnecessary variation. This isn't about constraining flexibility but about creating reliable foundations that enable customization when needed.
Protocol harmonization begins with collaborative partnerships between networks and collection centers, developing protocols that balance consistency with practical implementation. Through partnerships with manufacturers like Terumo, networks can leverage consistent platform technologies while maintaining flexibility for specific requirements.
Network-level coordination enables what individual centers cannot achieve alone. Centralized auditing ensures every site meets the same standards, while ongoing monitoring maintains performance over time. This approach has enabled networks to achieve starting material inconsistency rates as low as 1-4%, compared to industry averages that are significantly higher.
Comprehensive training at each collection center ensures personnel understand not just the procedures, but the rationale behind quality requirements and their downstream implications.

From Theory to Practice
Building and maintaining a standardized apheresis network requires more than identifying qualified centers. It demands ongoing relationship management, performance monitoring, and collaborative optimization. Networks like SCTbio's 150+ qualified collection sites represent years of relationship building, auditing, and protocol refinement.
The audit process itself becomes a competitive advantage, shortening the typically lengthy qualification timelines that can extend 6+ months when starting from scratch. Pre-qualified, regularly monitored centers enable rapid trial initiation while maintaining the highest quality standards.
Measurable outcomes include:
- Collection consistency: Networks achieve starting material variability rates of 1-4%
- Timeline improvements: Pre-qualified networks reduce center activation timelines significantly
- Manufacturing efficiency: Consistent starting materials enable optimized protocols and reduced batch failure rates
- Patient experience: Standardized procedures and trained staff improve patient comfort and collection success rates
Moving Forward
Organizations face a fundamental decision: build standardization capabilities internally or partner with established networks that have already solved these challenges. Industry experience suggests that many organizations recognize the value of partnering rather than building from scratch, reflecting the complexity and resource requirements of effective network management.
The choice isn't just about operational efficiency but about strategic focus. Every resource devoted to building apheresis capabilities is a resource not available for core therapeutic development.
High-quality starting materials remain the cornerstone of successful cell therapies, but achieving consistent quality doesn't require building these capabilities alone. Whether you're planning your first clinical trial or scaling toward commercial manufacturing, the foundation of success begins with reliable, consistent starting materials.
Related Resources
This blog is part of our ongoing series on optimizing apheresis operations and starting material quality. Dive deeper into the strategic considerations shaping the future of cell therapy development:
Want to discuss your apheresis challenges? Schedule a 15-minute consultation with our network specialists to explore how standardized partnerships could benefit your programs.


