SCTbio services

Full services covering GMP production, testing & logistics of advanced therapy medicinal products
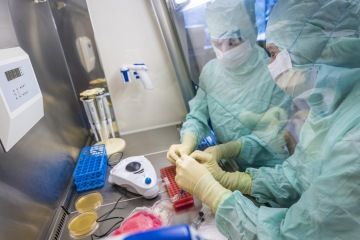
SCTbio delivers the highest quality collaboration through a range of services ensuring GMP compliance for the full life cycle of drug development, that include technology transfers, process development, clinical manufacturing services, fill & finish, quality control, QA/QP release, storage and logistics.
What we do
cGMP Manufacturing
& Quality Control
Analytical
Development
Process
Development
Logistics Services
& Apheresis Collection
Procurement
& Warehouse Management
Quality & Regulatory Support
& Qualified Person Services
Unique global network of Apheresis Centers
iin setting up the appropriate procedures within a network of 150 qualified and regularly monitored cell-collection sites.